16 Which One of the Following Formulas Represents an Aldehyde
CH 3 OH b Write the molecular formula of the third member of the homologous series of carbon compounds with general formula C n H 2n1 OH. As mentioned on one Reagent Friday back in the day ozone does more than absorb UV radiation in the upper atmosphere and cause breathing problems in traffic-clogged cities.
20 3 Aldehydes Ketones Carboxylic Acids And Esters Chemistry
I H2O ii CO2 iii CH4 Question 12 Calculate the mass per cent of different elements present in sodium sulphate Na2SO4.
. A i b i and iv c ii and iii d ii and iv - Get the answer to this question and access a vast question bank that is tailored for students. One of these substituents is the hydrogen atom and the other is an alkyl. A Propylbenzene b 3-Methylpentanenitrite c 2 5-Dimethylheptane d 3-Bromo- 3-chloroheptane e 3-Chloropropanal f 2 2-Dichloroethanol.
Gas is produced smoke or bubbles Steam is. A methyl group b aldehyde group c carboxyl group d magnesium. A CH 3 CH 2 CH 2 OH b CH 3 CHOHCH 3 c CH 3 CH 2 CH 2 CHO Note.
A Define a homologous series. Ozone O 3 Is A Powerful Oxidant For Cleaving Alkenes To Carbonyl Compounds. 2117 Markush Claims R-102019 I.
Balanced equations for each of the following reactions one using condensed formulas and one using Lewis structures. Which one occurs both during cyclic and noncyclic modes of photophosphorylation. Question 14 Calculate the amount of carbon dioxide.
Write two complete balanced equations for each of the following reactions one. What is the enthalpy change for the following reaction. C Name any two fossil fuels.
Ketone vs Aldehyde. NCERT Exemplar Solutions Class 10 Science Chapter 4 Free PDF Download. Which of the following compounds is a functional group isomer of C 2 H 5 OH ethanol ethyl alcohol.
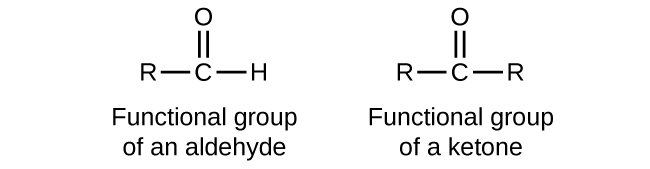
An aldehyde is a functional group which consists of two substituents attached to a carbonyl carbon. Which of the following ionic compounds will be the least soluble in water. Ex parte Markush 1925 Dec.
Which one of the following compounds is an isomer of CH 3 CH 2 CH 2 CH 2 OH. A Involvement of both PS I and PS II b Formation of ATP c. Isopropyl alcohol 23-Dimethylbutanal Heptan-4-one.
A Draw the structures for the following. The listing of specified alternatives within a Markush claim is referred to as a Markush group or Markush grouping. Write bond-line formulas for.
Organic Chemistry Test Answers A comprehensive database of more than 28 organic chemistry quizzes online test your knowledge with organic chemistry quiz questions. The equilibrium between the soluble ions and insoluble salt can be described by the solubility. I 2 moles of H 2 0 ii 20 moles of water iii 6022 1023 molecules of water iv 120441025 molecules of water Choose the option.
Question 5Which of the following represents the correct TUPAC name. This is one way to write an aldehyde d CH 3 CH 2 CH 2 CH 3 e none of the above 6. Give the TUPAC names of the following compounds.
In re Harnisch 631 F2d 716 719-20 CCPA 1980. Question 13 Determine the empirical formula of an oxide of iron which has 699 iron and 301 dioxygen by mass. Studying this NCERT will help you make your foundation strong and you can easily understand.
Com Categories ALL Organic Chemistry 2 Chapter 19-21 Questions Test 3 Tags C. Which of the following correctly represents 360 g of water. Question 11 Calculate the molecular mass of the following.
NCERT Exemplar Class 10 Science Chapter 4 Carbon and Its Compounds is an important study material required by students to gain abundant knowledge on the topic covered in CBSE Class 10 Chapter 4 syllabus. MARKUSH CLAIM A Markush claim recites a list of alternatively useable members. A ethanal CH 3 CHO b.
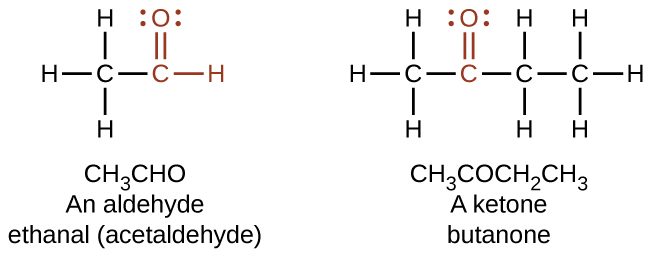
The study of compounds that contain the element carbon. The names for aldehyde and ketone compounds are derived. Ethanol reacts with propionic acid.
Give the name and structural formula of one homologue of the following. PBF2 Ksp 33 x 10 8 PBF2 Ksp 33 x 10 8 A. 15 Three of the following displayed formulae represent the same isomer of C3H4Cl2 but one structure represents a different isomer X.
Identify the structure of the major product formed in the following reaction and give a mechanism of its formation. Benzoic acid C 6 H 5 CO 2 H is added to a solution of sodium hydroxide. Its a powerful oxidant and since its discovery in the mid 1800s by Schönbein has found use in the cleavage.
In this edition the experiments designed for a first course in. Chlorophyll a molecule at its carbon atom 3 of the Pyrrole ring II has one of the following.
Chapter 3 Aldehydes Ketones Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library

Functional Groups Ck 12 Foundation

20 3 Aldehydes Ketones Carboxylic Acids And Esters Chemistry
Comments
Post a Comment